Natural Inorganic Pigments are extracted from the earth bed and are
available in natural form like ochers, umbers etc.
The different kinds of Natural Inorganic Pigments are:
- Azurite- Actually these kind of natural inorganic pigments
are copper carbonates having greenish blue shading. From a very long
period, azurite has been using as a pigment. But, often these pigments
have been replaced by synthetic pigments or used to paint the expensive
ultramarine as under paintings.
- Red earths- These pigments are the most diverse kind of
natural inorganic pigments. These are made from clays and they have a
large amount of iron oxide. The color varies from dull yellow to dull
deep yellow or from dull orange to dull red or from dull dark brown to
dark brown.
- Yellow earths- These are natural earth containing silica and
clay. These pigments are present in hydrous form of iron oxide. These
pigments also contain gypsum or manganese carbonate. In all over the
world, these pigments are available and have been using from the
prehistoric period.
Synthetic Inorganic Pigments
Synthetic inorganic pigments are manufactured in the laboratory. These
pigments consists of metallic compounds like manganese violet, cobalt blue.
As these pigments are manufactured in the laboratory, so they are found in
pure form having fine particles. Synthetic inorganic pigments can also be
produced by the replication of the natural earth colors like Mars Red or
Yellow.
Natural Pigments
Naturally occurring pigments such as Cochineal, Aeppo galls, Annatto,
Indigo, Ochres and Iron Oxides are known as Natural Pigments. These pigments
have been used as colorants since prehistoric times. These pigments are
obtained from insects and plants and used in cosmetics. They have affinity
to all such foods and drugs which require color additives.
Natural pigments are good for use in the shower gel, bath bombs, bath
salts, shampoo, soap, lotion and in many more other products. These pigments
have good quality of bleed protectiveness in soap and these are also water
dispersible. But, these pigments don't have high intensity in light.
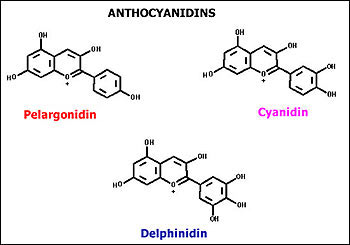
Anthocyanin Pigments- Anthocyanin pigments are colored pigments and
these are found profusely in plant kingdom. The colors imparted by these
pigments are blue, red and purple. The color of fruits and flowers are also
due to the color impartation of these pigments. Anthocyanins are soluble in
water and their extraction from the plant parts is also easy. Slightly acid
mixed water is required for the extraction of these pigments.
Carotenoid Pigmets- Carotenoid pigments have color range from yellow
to red. Mixture of acetone and hexane in the 1:3 ratio is used for the
extraction of carotenoid pigments. The acetone layer on the extracted
carotenoid pigments is removed with water. To remove the hexane residue,
make treatment of hexane residue with activated MgO2 diatomaceous earth
column. In this way, remaining of hexane and acetone can also be removed.
Yes! I am Interested
Annatto pigments are given 'annatto' name because
these pigments are derived from Annato shrubs. These pigments have
reddish-orange colorant. This colorant is derived from seeds of Achiote
trees. The concentration of annatto pigments in the annatto shrubs, vary
from place to place.
Uses of Annatto Pigments
When the foodstuffs require hues of yellow to orange, annatto based
pigments are used. The main food items in which annatto dyes are used are:
in making cheese (around 50%), for fish processing (around 20%),
confectionery (around 10%), diary products except cheese (around 20%).
Apart from the above mentioned natural pigment,
there are some more types of natural pigments present:
Betalain Pigments- Betalains are the another type of color pigments
which are also derived from plants. These pigments are present in two forms.
The first is b-cyanin having purple-red color and in the high concentration.
The second is b-xanthine which is yellow in color and in low concentration.
These pigments can easily be extracted from plant tissues with water as
these pigments are highly soluble in water. The extracted water is then
mixed smoothly with ethyl alcohol in 1:1 ratio. The use of ethanol in the
mixture is to reduce the enzymatic action, otherwise the pigments will be
degraded.
Plant pigment like chlorophyll- Acetone is required for the
extraction of plant pigments. Calcium carbonate must be present there for
stimulating the extraction process. Any other mild alkali can also be used
for the extraction of plant pigments. The alkali is used during the
extraction process due to neutralizing the acid which liberates from the
plant tissues. This neutralization prevents the formation of pheophytins
during the extraction process, unless the the pheophytins may block the
extraction process. Following diagram is the pigment structure of
anthocyanidin pigments (flower pigments).

![]() Profile
Profile ![]() Product Range
Product Range![]() Industries
Industries![]() Infrastructure
Infrastructure![]() Our Quality
Our Quality![]() Custom Manufacturing
Custom Manufacturing![]() Network
Network![]() Contact Us
Contact Us![]() Send Enquiry
Send Enquiry


![]()

![]() Profile
Profile
![]() Product
Range
Product
Range![]() Industries
Industries![]() Infrastructure
Infrastructure![]() Our
Quality
Our
Quality![]() Custom
Manufacturing
Custom
Manufacturing![]() Network
Network![]() Contact
Us
Contact
Us![]() Send
Enquiry
Send
Enquiry